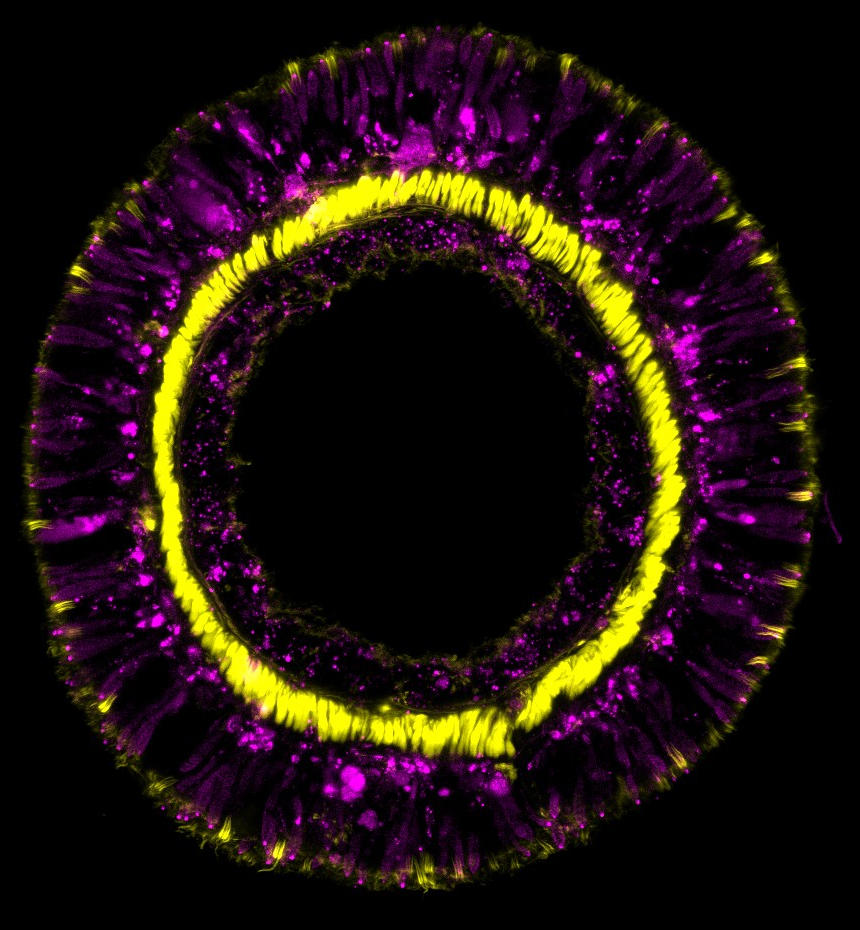
Cross-section through a tentacle of a transgenic sea anemone showing differentiation products of the SoxC cell population (magenta) and retractor muscles (yellow). Credit: Andreas Denner
In sea anemones, highly conserved genes guarantee the lifelong differentiation of neurons and glandular cells.
Sea anemones are seemingly immortal animals. They seem to be immune to aging and the negative impacts that humans experience over time. However, the exact reasons for their eternal youth are not completely understood.
The genetic fingerprint of the sea anemone Nematostella vectensis reveals that members of this incredibly ancient animal phylum employ the same gene cascades for neural cell differentiation as more complex organisms. These genes are also in charge of maintaining the balance of all cells in the organism during the anemone’s lifetime. These findings were recently published in the journal Cell Reports by a group of developmental biologists headed by Ulrich Technau of the University of Vienna.
Almost all animal organisms are made up of millions, if not billions, of cells that join together in intricate ways to create specific tissues and organs, which are made up of a range of cell types, such as a variety of neurons and gland cells. However, it is unclear how this critical balance of diverse cell types emerges, how it is regulated, and if the different cell types of different animal organisms have a common origin.

Optical longitudinal section of a sea anemone with nanos1-transgenic neuronal cells (red) in both cell layers. Muscles are stained green, cell nuclei in blue. Credit: Andreas Denner
Single-cell fingerprint leads to common ancestors
The research group, led by evolutionary developmental biologist Ulrich Technau, who is also head of the Single Cell Regulation of Stem Cells (SinCeReSt) research platform at the University of Vienna, has deciphered the diversity and evolution of all nerve and gland cell types and their developmental origins in the sea anemone Nematostella vectensis.
In order to achieve this, they used single cell transcriptomics, a method that has revolutionized biomedicine and evolutionary biology over the past decade.
“With this, entire organisms can be resolved into single cells – and the entirety of all currently expressed genes in each individual cell can be decoded. Different cell types fundamentally differ in the genes they express. Therefore, single cell transcriptomics can be used to determine the molecular fingerprint of each individual cell,” explains Julia Steger, the first author of the current publication.
In the study, cells with an overlapping fingerprint were grouped. This allowed the scientists to distinguish defined cell types or cells in transitional stages of development, each with unique expression combinations. It also allowed the researchers to identify the common progenitor and stem cell populations of the different tissues.
To their surprise, they found that contrary to earlier assumptions, neurons, glandular cells, and other sensory cells originate from one common progenitor population, which could be verified by genetic labeling in living animals. Since some gland cells with neuronal functions are also known in vertebrates, this could indicate a very old evolutionary relationship between gland cells and neurons.
Ancient genes in constant use
One gene plays a special role in the development of these common ancestor cells. SoxC is expressed in all precursor cells of neurons, gland cells, and cnidocytes and is essential for the formation of all these cell types, as the authors were additionally able to show in knockout experiments.
“Interestingly, this gene is no stranger: It also plays an important role in the formation of the nervous system in humans and many other animals, which, together with other data, shows that these key regulatory mechanisms of nerve cell differentiation seem to be conserved across the animal kingdom,” says Technau.
By comparing different life stages, the authors also found that in sea anemones, the genetic processes of neuron development are maintained from the embryo to the adult organism, therefore contributing to the balance of neurons throughout the life of Nematostella Vectensis.
This is remarkable because, unlike humans, sea anemones can replace missing or damaged neurons throughout their lives. For future research, this raises the question of how the sea anemone manages to maintain these mechanisms, which in more complex organisms only occur in the embryonic stage, into the adult organism in a controlled manner.
Reference: “Single-cell transcriptomics identifies conserved regulators of neuroglandular lineages” by Julia Steger, Alison G. Cole, Andreas Denner, Tatiana Lebedeva, Grigory Genikhovich, Alexander Ries, Robert Reischl, Elisabeth Taudes, Mark Lassnig and Ulrich Technau, 20 September 2022, Cell Reports.
DOI: 10.1016/j.celrep.2022.111370
Share your story or advertise with us: Whatsapp: +2347068606071 Email: info@newspotng.com