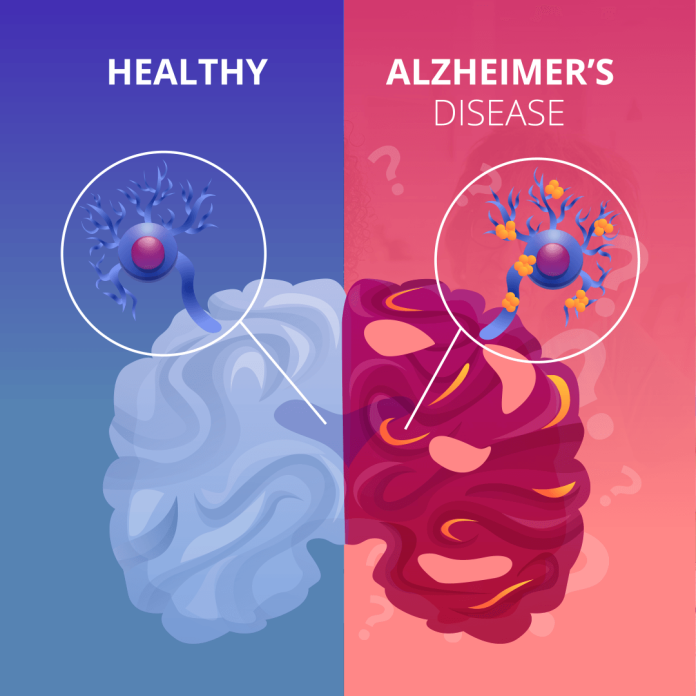
A new drug known as Donanemab tends to keep the hope of patients suffering from Alzheimer’s disease – a degenerative brain disease, alive as the drug is said to slow mental decline caused by the disease by 36 per cent.
According to the Daily Mail, the drug could spell ‘the beginning of the end’ for the degenerative brain disease.
In the initial findings published on May 3, the drug, when taken as a monthly infusion for 18 months, halted a reduction in the ability to perform daily activities by up to 40 per cent.
But the US pharmaceutical giant, Eli Lilly, is expected to unveil the full results of its trials of the drug on Tuesday which according to the Daily Mail is the second shown to slow the progression of dementia.
Another drug, lecanemab, which was approved for use in the United States on July 7, was found to have also reduced the cognitive decline among those with the memory-robbing condition by 27 per cent with the United Kingdom on the brink of introducing the drug.
“After 20 years with no new Alzheimer’s disease drugs in the UK, we now have two potential new drugs in 12 months,” the Associate Director of Alzheimer’s Society, Dr Richard Oakley, said while writing for The Mirror.
According to the Daily Mail, Donanemab works by clearing plaque clusters from the brain known as amyloid, closely associated with Alzheimer’s disease.
The drug is said to be administered to patients via once-a-month intravenous injections for up to 18 months, or until the amyloid clusters in the brain had been cleared.
In a phase three trial, Donanemab slowed down the decline in patients’ ability to think clearly and perform daily tasks by 36 per cent compared to a placebo.
And tomorrow, experts across the world would begin to measure the risks of the groundbreaking drug against the benefits, as such drugs could carry risk factors such as brain swelling and bleeding, the Daily Mail reports.
Share your story or advertise with us: Whatsapp: +2347068606071 Email: info@newspotng.com