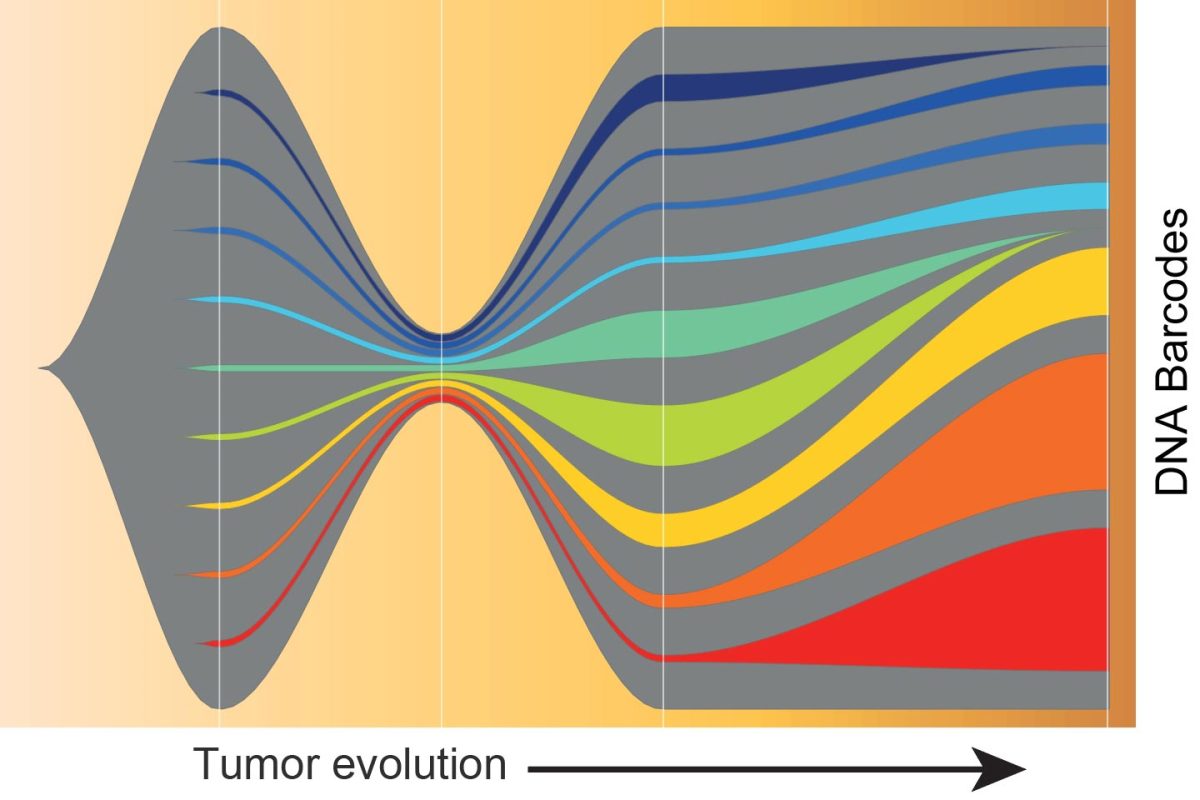
Each colored ribbon represents a DNA barcode. As the tumor evolves, some cancer cells come to dominate the tumor, shown by the orange and red ribbons. This shows these cancer cells have a pre-existing ability to escape the immune system and can carry on growing, even after treatment. Credit: Garvan Institute of Medical Research
Using DNA barcodes to track cancer cells through time, scientists show that cells within a cancer have diverse abilities to escape immune system defenses.
According to a new scientific study from the Garvan Institute of Medical Research, some cancer cells can deploy parallel mechanisms to evade the immune system’s defenses as well as resist immunotherapy treatment.
Breast cancer cells are able to replicate and metastasize by suppressing the action of killer T-cells and hindering the ability of the immune system to flag tumor cells for destruction, the researchers found.
“We know that breast cancer typically doesn’t respond well to immunotherapy, and we wondered if there’s an intrinsic mechanism enabling breast cancer cells to escape the immune system,” says first author Ms. Louise Baldwin, who is a PhD student in Associate Professor Alex Swarbrick’s lab at Garvan.
For the research, the scientists used a technique called DNA barcoding, which tags cells with a known sequence and tracks the progression of tumor cells through time.
“We showed that there are rare cancer cells capable of escaping the immune system and escaping treatment with immunotherapy,” Ms. Baldwin says.
The mechanisms could be used as potential targets for therapies, to stop tumorous cells from adapting and spreading. Another future application could be in prognosis, where a high number of cells could indicate which patients might not respond sufficiently to immunotherapy.
The new study will be published today (November 7) in the journal Nature Communications.
In this video, Professor Alex Swarbrick explains the research.
Although immunotherapy is an effective treatment for many cancers, in some people their cancer cells evolve to outplay the immune system defenses. This process is known as immunoediting. It is where interactions between tumor cells and immune cells result in many cancerous cells being destroyed by the immune system, but some are left undetected, which continue to grow and spread.
Mouse breast cancer cells tagged with a known DNA ‘barcode’, a sequence that was passed on from one generation of cells to the next, were used by the researchers.
The barcoding allowed the team to locate where more aggressive, resistant cells came from, as they could trace it back to the original cell to see if it had grown or shrunk.
“Lead author Dr. Simon Junankar wanted to understand whether resistance was adaptive – whether cancer cells duck and weave – or are they pre-programmed to evade the immune system,” says Associate Professor Alex Swarbrick, a laboratory head and Co-Lead of the Dynamic Cellular Ecosystems in Cancer Program at Garvan.

Professor Alex Swarbrick. Credit: Garvan Institute of Medical Research
The research team discovered that even before treatment, the cancer cells had diversified. “Some cells had already acquired the ability to evade immunity, meaning they have an innate ability to escape the immune system,” he says.
The cells seem to do this with parallel approaches. One way is to suppress the action of killer T-cells, which would usually destroy harmful cells. The other is to reduce the expression of MHC1 on cells, which acts as a flag for the immune system to recognize harmful cells.
“Most tumor cells vanish when the immune system gets switched on, but a small proportion keeps growing and expanding,” says Associate Professor Swarbrick.
“Tumors keep evolving and diversifying, and action by the immune system or treatment like chemotherapy is like pruning a tree – cancer cells get wiped out but the remaining branches on the tree continue to grow.”
The scientists also investigated the genetics of the cells, but there were no genes found to be associated. This suggests that epigenetics might be at play.
Reference: “DNA barcoding reveals ongoing immunoediting of clonal cancer populations during metastatic progression and immunotherapy response” 7 November 2022, Nature Communications.
DOI: 10.1038/s41467-022-34041-x
Associate Professor Swarbrick is a Conjoint Associate Professor at St Vincent’s Clinical School, Faculty of Medicine and Health, UNSW Sydney.
This research was supported by research grants from the National Breast Cancer Foundation (NBCF). Louise Baldwin is supported by an Australian Government research training (RTP) stipend and Associate Professor Swarbrick is the recipient of a research fellowship from the NHMRC.
Share your story or advertise with us: Whatsapp: +2347068606071 Email: info@newspotng.com











 NEWSPOT NIGERIA DAILY PULSE
NEWSPOT NIGERIA DAILY PULSE  May 07, 2025
May 07, 2025




