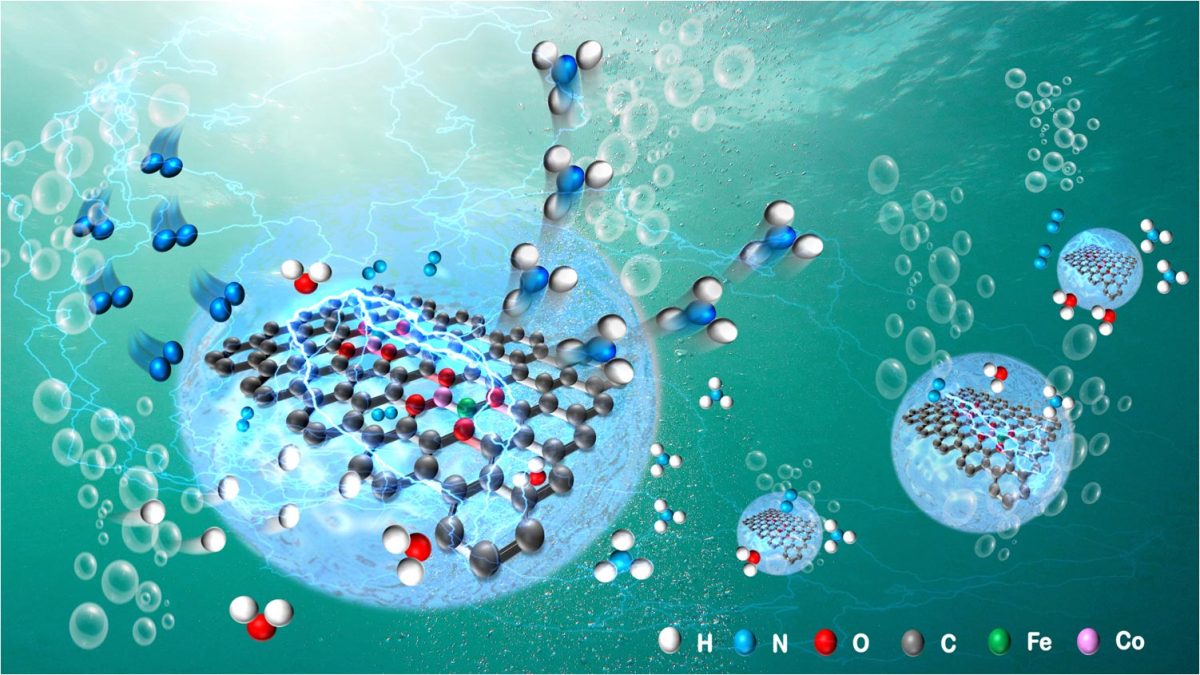
Schematic diagram of ammonia synthesis by electrocatalysis with bimetallic Fe–Co single-atom catalyst. Credit: Shengbo Zhang
Scientists have demonstrated the use of controllably synthesized single-atom catalysts (SACs) to depict the relationship between electrocatalytic nitrogen reduction reaction (NRR) performance and single-atom (SA) loading.
Conducted by researchers from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences, the study will be published today (November 14) in the journal Nature Sustainability.
Electrosynthesis of ammonia from NRR at ambient conditions has been widely regarded as a “green ammonia synthesis” technology to replace the traditional energy- and capital-intensive Haber-Bosch process.
Scientists agree that the intriguing features of SACs may create a new catalytic paradigm. However, one of the key challenges hindering the rational design and development of SACs is the lack of insight into the relationship between performance and SA loading, due mainly to the inability to precisely control the synthesis of SACs with desired SA loading densities and active site coordination forms.

(a) HAADF-STEM images. (b) Plots of the loaded Fe and Co SAs against [Fe3+] and [Co2+]. (c) Fe and Co K edge EXAFS fitting curves. (d) Co K edge XANES spectra. (e) Solid-state 13C NMR spectra. (f) RNH3 and FE of corresponding catalysts. (g) Effect of catalyst loading density on the geometric area activity. (h) DFT optimized configurations. (i) DFT optimized configurations after the initial electrocatalytic NRR cycle. Credit: Shengbo Zhang
In this study, the researchers demonstrated an adsorption-regulated synthetic method that uses bacterial cellulose as an adsorption regulator to control Fe3+/Co2+ impregnation on bacterial cellulose through carbothermal reduction. Fe–Co SAs were then fixed to bacterial cellulose-derived carbon via bimetallic [(O–C2)3Fe–Co(O–C2)3] coordination.
Importantly, the scientists unveiled a suite of relationships that quantitatively define Fe3+/Co2+ distribution between bacterial cellulose and the adsorption solution, and the percentage conversion of impregnated Fe3+/Co2+ on bacterial cellulose to Fe/Co SAs on bacterial cellulose-derived carbon. They then demonstrated the use of such quantitative relationships to guide the controllable synthesis of bimetallic Fe–Co SACs with desired Fe/Co contents and atomic ratios.
They showed that controllably synthesized SACs can depict the electrocatalytic relationship between NRR performance and SA loading. Single-atom electrocatalysts (SAECs) with a unity Fe/Co atomic ratio possess the highest site density and NRR performance for bimetallic Fe–Co SAs, making them capable of achieving a superb ammonia yield rate with exceptional faradaic efficiency.
The catalytic activity of SACs, in contrast with other types of catalysts, is determined by the nature of the SA, the physiochemical properties of the support and, importantly, the coordination bonds that anchor the SA to the support.
Under electrocatalytic NRR conditions, [(O–C2)3Fe–Co(O–C2)3] in the as-synthesized bimetallic Fe–Co SAECs is operando transformed into the more stable coordination configuration [(O–C2)3Fe–Co(O–C)C2], thus promoting and sustaining NRR performance.
The researchers suggest that these new findings will be of great interest to the broad catalysis community.
Reference: “Atomically dispersed bimetallic Fe–Co electrocatalysts for green production of ammonia” 14 November 2022, Nature Sustainability.
DOI: 10.1038/s41893-022-00993-7
Share your story or advertise with us: Whatsapp: +2347068606071 Email: info@newspotng.com